QAtrain
Provides Automated training plans
QAtrain has been released for customer implementation. QAtrain is QAtor’s electronic compliance solution for handling training plans and automatic notification of needed training or retraining of users.
The solution will be delivered including validation documentation according to FDA-GxP leading to fast implementation, ready-to-use and is standard-integrated with other QAtor compliance solutions, e.g QAchange – QAtor’s solution for electronically handling changes and deviations.
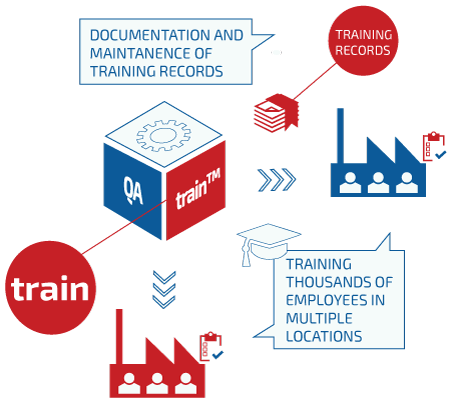
QAtrain™can be used as an independent product, interfacing only with QAtor support modules providing notifications, storage, security, privileges, etc. QAtrain™ can also work in conjunction with other QAtor products such as QAcii™ , QAchange™ or other QAtor suite products.

Making sure that employees are properly trained to correctly perform their tasks
Supporting electronic re-training based on configuration

Providing documented proof of planned and systematic training processes

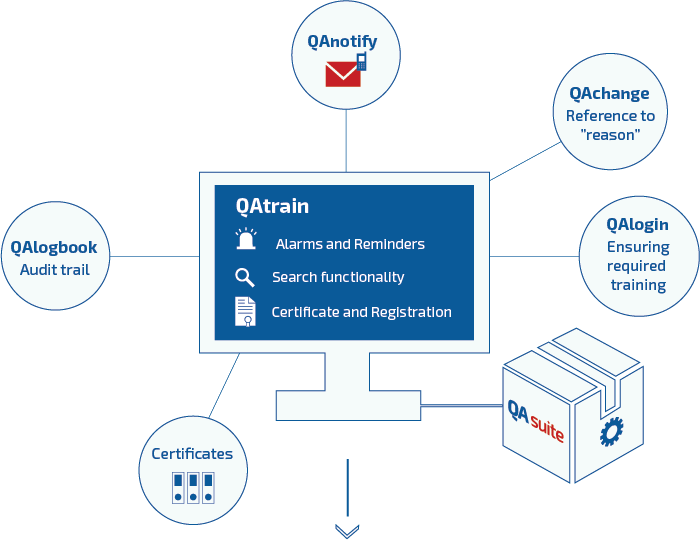
The QAtrain™ tool is like all Qator web based applications and meets the requirements expected in the pharmaceutical industry (GxP, FDA requirements in general, Eudralex etc.). The system secures traceability in the entire process. The system fulfills the FDA 21 CFR part 11 requirements regarding signatures and audit trails as well as EEC annex 11 cGxP standards. Data stored in QAtrain™ (or links to data on GxP drives) must be filed in a database and subject to full audit trail logging and access control.

Set up Training Plans that specific groups must read
Let users have an easy overview of their missing training documentation


Let users sign for read SOPs and let trainers sign for performed user training
Enjoy the system having an overview of the training compliance

Key Benefits
DIGITAL SIGNATURES
FDA AND GMP COMPLIANT
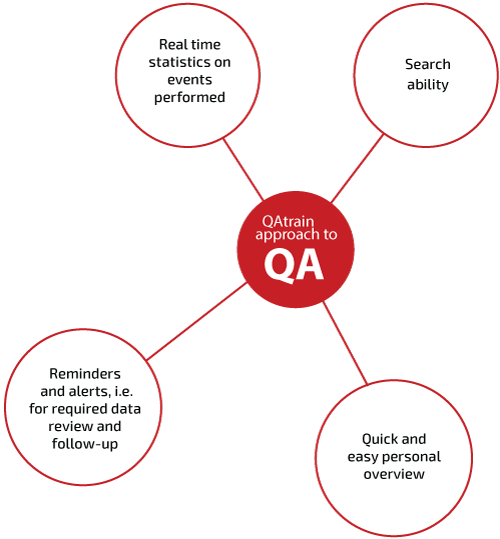
STATISTICAL REPORTS
WEB BASED EASY- TO-USE INTERFACE
More Features
- Control of individual training plans incl. current state
- Auto generation of reading lists based on training plans
- Configurable structure to match company
- Organizational units and project overview with compliance level
System Requirements

TO TOP
Over 50 years of combined life science and Quality Assurance experience within Qator A/S.
Applying the newest tools and methods to enhance your company’s success, by improving compliance and traceability.
Possibility of intergration with other systems.
Laurentsvej 27, 2880 Bagsværd, Denmark
+45 70 27 83 27


