QAbaseline
Provides an electronic file modification tracker
FDA regulative 211.184 (c)
“(c) An individual inventory record of each component, drug product container, and closure and, for each component, a reconciliation of the use of each lot of such component.
The inventory record shall contain sufficient information to allow determination of any batch or lot of drug product associated with the use of each component, drug product container, and closure.”
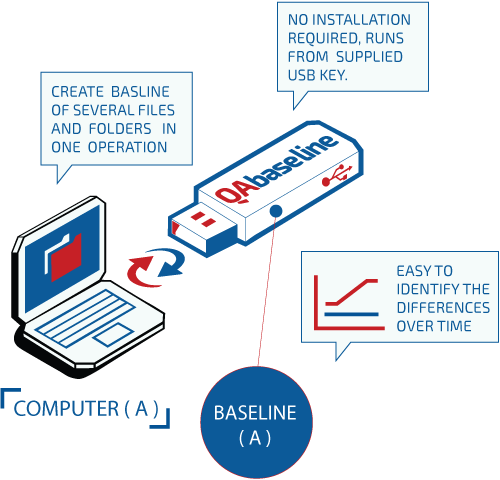
QAbaseline is a tool for creating baselines of several files in just one operation. It can compare a baseline with previous baselines and clearly identify removed, added files or modified files in a compare report.

QAbaseline Key Benefits

Save baselines in
a single file, making
it easier to manage

FDA and GMP
compliant

Easy and simple
user interface
Easy to perform,
manage
and compare baselines
on files
Save settings for
baselines to perform
them again and again
No installation
required, runs from
supplied USB key
Baseline data will
be saved on USB key,
so target machine is
not altered

Can run on
640 / 480
QAbaseline is a tool developed and validated by QAtor for keeping track of large numbers of files and easily identify differences over time using a well-defined mathematical algorithm (HASH function) which converts a large, possibly variable-sized amount of data, into a single number.
TO TOP
Over 50 years of combined life science and Quality Assurance experience within Qator A/S.
Applying the newest tools and methods to enhance your company’s success, by improving compliance and traceability.
Possibility of intergration with other systems.
Laurentsvej 27, 2880 Bagsværd, Denmark
+45 70 27 83 27


