QAchange
Provides a simple & easy to use E-compliance tool.
The authorities require that you as a Life Science company have a working written Change Control and Deviation Handling procedure

FDA regulative 211.100
“There shall be written procedures for production and process control designed to assure that the drug products have the identity, strength, quality, and purity they purport or are represented to possess. Such procedures shall include all requirements in this subpart.
These written procedures, including any changes, shall be drafted, reviewed, and approved by the appropriate organizational units and reviewed and approved by the quality control unit. (b) Written production and process control procedures shall be followed in the execution of the various production and process control functions and shall be documented at the time of performance. Any deviation from the written procedures shall be recorded and justified”.
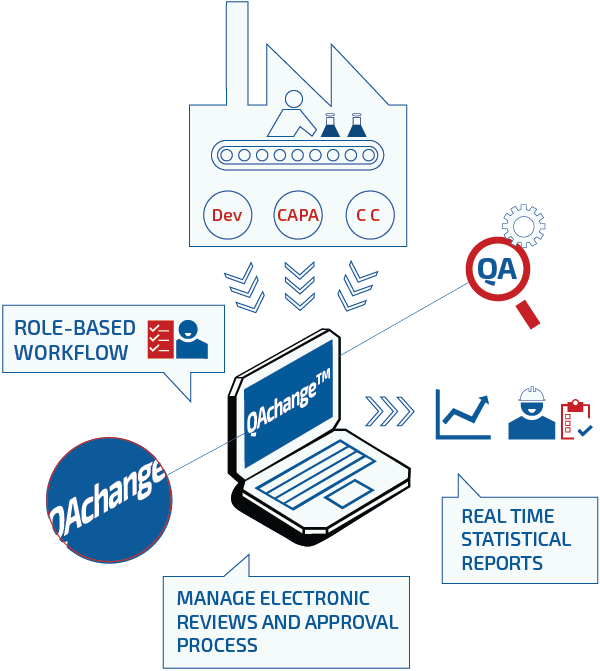
Most deviations are due to various reasons not handled 100%, according to the procedures. Often document signatures are made by people having the wrong roles or not participating in the according project. It is also often seen that signatures fall in a timely disorder or are delayed waiting for the correct people to sign off at a specific phase in the documents life-cycle.
Solution
QAchange™ automates the Change Control and deviation process via an intelligent Web-based interface. It supplies fully automated handling of the workflow for any given deviation or Change Control Case [CC], and through customizable templates that relates roles to specific tasks. Any template workflow defined by customer SOPs can be implemented in the systems templates, all made easy accessible where needed via the zero client Web-interface.
QAchange™ can also work in conjunction with other QAtor suite products.
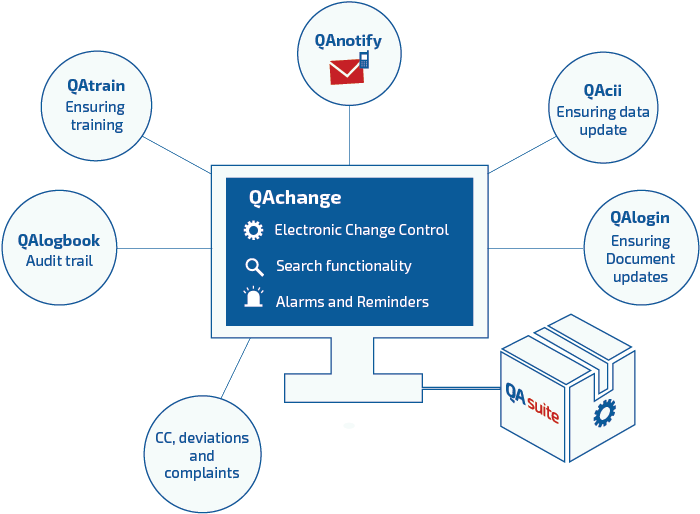
Manageability
All this is unnecessary document disorder and delay. GMP is all about planning ahead and showing control. This is what QAchange™ enables you to do:

Electronic change control process ensures not only savings in time and cost but also better collaboration, visibility for management and indisputable audit trails of electronic signatures in compliance with FDA’s 21 CFR Part 11. QAchange™ furthermore secures traceability in the entire process.
Functionality
With its focused industry templates and protocols, electronic signature functionality, change control processes, integrated workflow, and security features, the Quality Assurance suite is particularly well suited for companies that need to enforce regulatory compliance with strict quality control measures. By providing exceptional visibility and transparency, control and management, the solutions enable companies to more efficiently manage compliance requirements through controlled electronic repositories, process automation, collaboration, communications, archival, and business integration capabilities in audited and tightly managed environments.

Quickly discover the impact of change and lower your total cost of quality while ensuring that GxP is kept at the highest level at all time
Key Benefits
SHARED
DATABASE
FDA AND GMP COMPLIANT
STATISTICAL REPORTS
WEB BASED EASY- TO-USE INTERFACE
More Features
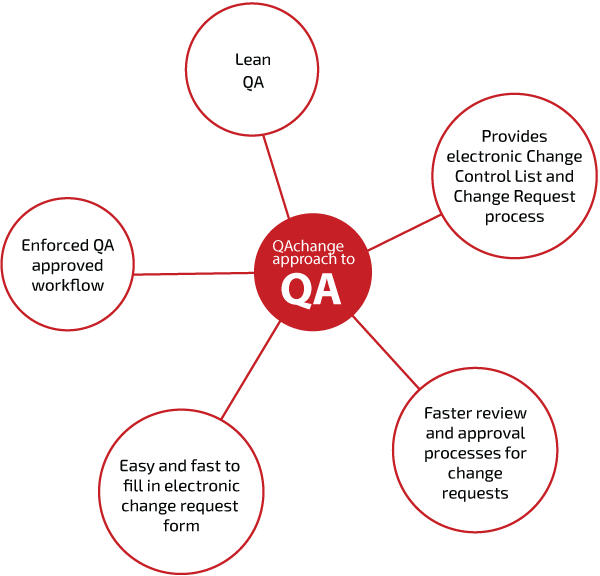
- Customizable forms with enforcement of required fields
- Manages electronic reviews and approval process with the workflow engine
- Ensures full control and audit trail
- Improves quality, adds transparency and address regulatory concerns
System Requirements

TO TOP
Over 50 years of combined life science and Quality Assurance experience within Qator A/S.
Applying the newest tools and methods to enhance your company’s success, by improving compliance and traceability.
Possibility of intergration with other systems.
Laurentsvej 27, 2880 Bagsværd, Denmark
+45 70 27 83 27


