QAlogbook
Provides a simple & easy to use Electronic Logbook tool
FDA regulative 211.182
“If equipment is dedicated to manufacture of one product, then individual equipment logs are not required, provided that lots or batches of such product follow in numerical order and are manufactured in numerical sequence.
In case where dedicated equipment is employed, the records of cleaning, maintenance and use shall be part of the batch record. The persons performing and double-check in the cleaning and maintenance shall date and sign or initial the log indicating that the work was performed. Entries in the log shall be in chronological order”.
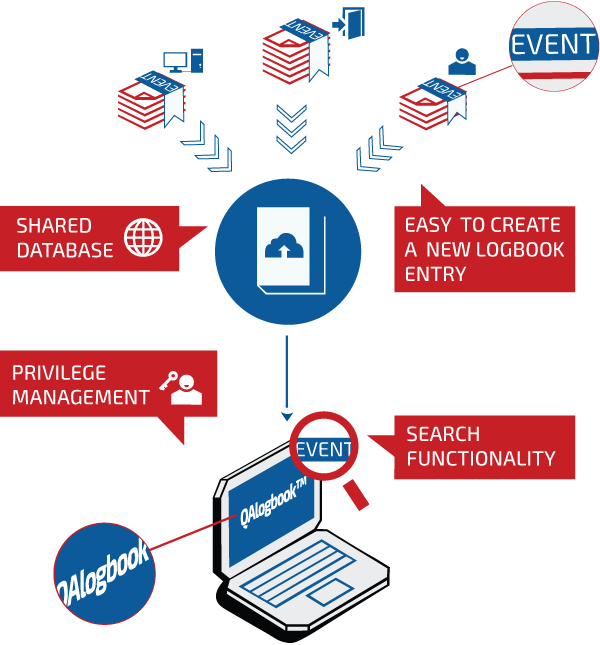
FDA Compliant Method
The Web-based application utilizes true 21 CFR part 11 compliant electronic signatures, multitiered privilege levels and time-stamped audit trails to ensure data integrity for legal and regulatory purposes, eliminating tampering risks that until now have forced Life Sciences to maintain handwritten documentation of equipment cleaning, maintenance and use.
Functionality
QAlogbook™ provides the functionality to capture and transform logbook entries into operational intelligence, making the data accessible across the organization to a wide variety of users. It replaces the myriad of paper logs, spreadsheets and disparate databases to integrate information from many different sources in one location.
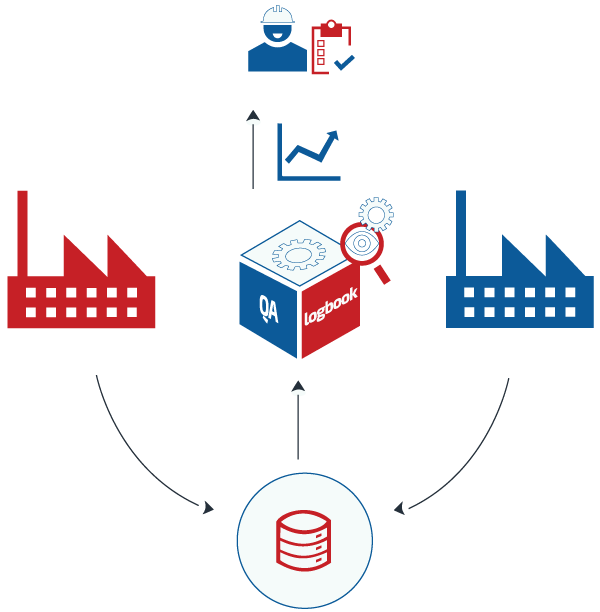
Manageability
The application enhances the ability of logbooks to act as a source of management information and records key site status information on environment, equipment and other issues to the resources responsible for support and maintenance. Information may be required both, on a daily basis to understand the current equipment condition, or issues and also historically to search for historical events and build up evidence of trends. The electronic logbook provides the ability to search, view and report on historic equipment data for event tracking and in support of trend analysis. The logbook has a quick and intuitive method to enter data, which ensures that time spent identifying, finally writing logs and capturing operational information is minimized.

Easy logbook Configuration
Whilst providing a mechanism for improved consistency of logs and reports in terms of format, structure and content.
QAlogbook™ allows the user complete flexibility in being able to configure log entries of exactly the data needed according to instructions, including text fields, time and date, drop-downs , radio- and checkboxes.
Key Benefits

PRIVILEGE MANAGEMENT
FDA AND GMP COMPLIANT
STATISTICAL REPORTS
WEB BASED EASY- TO-USE INTERFACE
More Features
- Pre-validated application (FDA/GxP). Documentation comes as part of deliverance
- Seamless integration with other QAsuite™ applications
- Configurable logbook layout, i.e. customizable forms, check boxes, drop down menus with pre-defined text, and with enforcement of required fields and the option to upload attachments
- Standardized logbook entries with real-time electronic logbook management (creating and maintaining equipment data)
System Requirements

TO TOP
Over 50 years of combined life science and Quality Assurance experience within Qator A/S.
Applying the newest tools and methods to enhance your company’s success, by improving compliance and traceability.
Possibility of intergration with other systems.
Laurentsvej 27, 2880 Bagsværd, Denmark
+45 70 27 83 27


